What is the NGOR?
The National Gynae-Oncology Registry (NGOR) is a clinical quality registry for gynaecological cancers, led by Professor John Zalcberg, an oncologist and cancer researcher, and Associate Professor Robert Rome, a gynaecological oncologist. The registry is operated by a team of researchers within Monash University’s School of Public Health and Preventive Medicine (SPHPM).
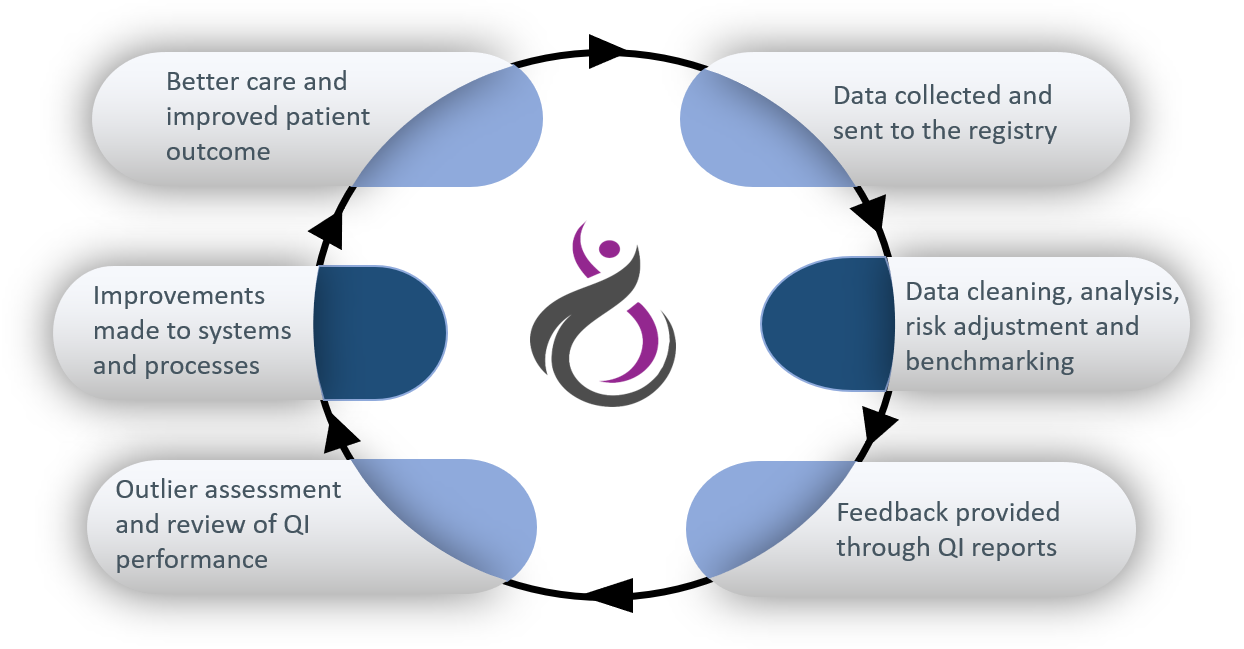
A clinical quality registry (CQR) is a database that systematically collects health information about people with a particular disease (in this case gynaecological cancers) to monitor their outcomes and report on the quality of the care that is provided to them. CQRs measure and monitor how closely care provided to Australian patients aligns with international experience and evidence-based practice guidelines. CQRs can be used to identify significant variation in care and outcomes, and drive improvements in practice. You can read more information about clinical quality registries on the Australian Commission of Safety and Quality in Healthcare website.
The National Gynae-Oncology Registry is divided into several modules, with each module based on the tumour’s anatomical location. Most tumour cell types will be included in each module, but due to differences in treatment, prognosis and management, some rarer subtypes may be excluded.
Data Collection
The registry gathers information about the diagnosis, treatment and outcomes of women with gynaecological cancers.
Currently, data are collected about the type of cancer and how it was diagnosed, how it was treated (including surgeries and surgical complications), and about various outcomes, such as recurrence and survival. These data are used to measure and monitor the overall quality of care given to women with gynaecological cancers and to report these measures back to clinicians and hospitals so that care can be improved.
Patient-reported outcome measures (PROMs) are questionnaires completed by patients, which ask how the disease itself, health services and interventions have affected their quality of life. PROMs provide data on important outcomes that might otherwise not be reflected in clinical databases or medical records. For example, they may ask about pain, appetite and impact on psychological health, distress and social roles. PROMs are currently in development for the NGOR, and we expect to begin implementing them in the near future.
Modules
Ovarian Cancer Registry (OvCR)
The most developed of the NGOR’s modules, it was piloted between 2017 and 2019, and has resulted in the recruitment of more than 1,500 patients with newly-diagnosed ovarian, tubal and peritoneal cancers.
Rare Ovarian Tumours Module
As a sub-module of the OvCR, it was developed in late 2021. Over 100 patients with non-epithelial ovarian cancer have been recruited thus far.
Endometrial Cancer Module
This module commenced its pilot phase in 2021 with the support of an Epworth Medical Foundation grant. It has now completed its pilot phase, with over 1,000 patients recruited.
Cervical and Vulvar Cancer Modules
These modules are currently under development and set to commence their respective pilot phase’s in the near future.
Funding
The NGOR pilot phase was generously supported by Ovarian Cancer Australia, the Australian Society of Gynaecologic Oncologists (ASGO) and the CASS Foundation.
The Audrey Voss Gynaecological Cancer Research Grant, awarded by the Epworth Medical Foundation allowed the registry to develop modules for endometrial, cervical and vulvar cancers. This project was focused on establishing a set of appropriate and meaningful quality indicators and datasets for each of the new tumour site modules (endometrial, cervical and vulvar cancers), and to pilot data collection in a stepwise fashion for each of these modules.
On May 8th 2020 (World Ovarian Cancer Day), the NGOR was one of eight research projects to be awarded funding from the Medical Research Future Fund (MRFF) to contribute to a greater understanding of ovarian cancer. This funding is being used to build upon the two year ovarian, tubal and peritoneal cancer pilot, by collecting clinical data and patient reported outcomes to provide risk-adjusted, benchmarked assessments of quality of care to healthcare providers. This funding will be provided from June 2020 to June 2025.
Information about Funding Bodies
Ovarian Cancer Australia is a patient advocacy group, providing women affected by ovarian, tubal and peritoneal cancers, and their families and friends, with information and support. They run face-to-face support groups, tele-support groups and an online forum which enables women to share their personal stories and connect with other women going through similar experiences. Ovarian Cancer Australia also provides Resilience Kits – free guides containing information on diagnosis, treatment, wellbeing and support for patients and their family, as well as links to useful resources. In addition to providing information and support to patients and their families, Ovarian Cancer Australia promotes a number of research efforts, including the NGOR’s ovarian, tubal and peritoneal cancer module. To order or download a Resilience Kit, or to access information, support and webinars, please visit their website or call 1300 660 334.
The CASS (Contributing to Australian Scholarship and Science) Foundation is a private philanthropic foundation which supports and promotes the advancement, research and practice of education, science and medicine.
The Australian Society of Gynaecologic Oncologists (ASGO) is a non-profit organisation established in 1986. The Society’s primary objective is to promote and improve standards of care for patients with gynaecological cancers. They also work to promote postgraduate, undergraduate and community education in the area of gynaecological oncology and to foster research into this area. ASGO members also advise and assist in the training and teaching of gynaecological oncologists.

